
Summary
Thin plate pure lead (TPPL) and lithium batteries are the two most advanced forklift battery technologies today. At their current state of development, TPPL batteries have a few disadvantages compared to the LFP version of Li-ion: lower energy density, lower maximum depth of discharge, and less-than-stellar performance at sub-zero temperatures. The higher price remains the lithium battery’s main disadvantage for smaller forklift fleets. Bigger companies invest in lithium batteries and see the ROI with their longer term of service and lower energy costs.
Looking ahead, the price of lithium-ion batteries will decrease with scale, while further engineering advances in TPPL batteries will make them more expensive and less attractive than Li-ion. Li-ion batteries’ share of motive power is expected to grow to nearly 50% of the market by 2030.
A basic description of lead-acid battery technology
TPPL technology shares the same engineering principle with all lead-acid batteries: lead plates are suspended in an electrolyte consisting of a water (H2O) and sulfuric acid (H2SO4) solution within a casing. Positive (cathode) and negative (anode) plates are always made of lead (Pb) alloy grid supports (current collectors) having dissimilar lead dioxide (PbO2) and lead coatings. As current flows between the plates due to the chemical reaction, lead sulfate forms on both the positive and negative plates. As the formation of lead sulfate increases, the voltage begins to decrease. The lead sulfate will crystallize upon the plates if a battery is not immediately connected to a charger and a charging current is applied.
The maximum open-circuit voltage that can be developed by a single lead-acid cell is 2.041 V.
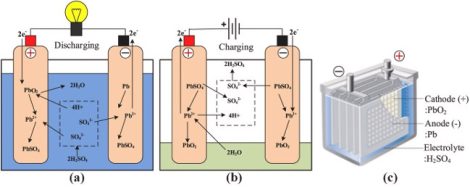

The latest developments in lead-acid battery technology
As lead-acid technology developed over the past few decades, new versions of the battery design were launched. The table below describes the various improvements in the technology, placing TPPL within the context of these lead-acid battery innovations.
Table 1. Lead acid battery sub-technologies available on the market.
| Development | Description | Nomenclature of lead-acid batteries |
|---|---|---|
| Container type | Lead-acid batteries produce hydrogen and oxygen gas (gassing) at the electrodes during charging. These gases entrain droplets of electrolyte, creating a sulfuric acid aerosol, which one can smell near a working forklift.
Gases are allowed to escape an open cell. Batteries with such cells are called vented lead-acid (VLA) batteries. However, the gases can be contained and recombined. Batteries such as sealed lead-acid (SLA) have a vent or valve to allow small portions of gases to escape. Valve-regulated lead-acid (VRLA) are SLA batteries with valves. |
|
| Electrolyte leakage protection | Batteries with liquid electrolytes are all flooded lead-acid (FLA) batteries. Any damage to a container inevitably leads to electrolyte leakage.
In many cases, for safer operation, the electrolyte can be physically forced to stay in a specified volume. Gelled or gel lead-acid batteries use special material that turns the electrolyte into a gel. Another way to bind electrolytes is to use an absorbent material. Batteries using such material between plates are called absorbed glass mat (AGM) batteries. |
|
| Shape of cell | In the past, battery packs with non-standard voltages were used widely. Using cylindrical cells, alternative battery configurations were easily constructed.
Today, the most widely used battery packs are rated at 6V and 12V. They have standard rectangular cells inside. |
|
| Usage of alloys in current collectors | Present-day collector grids are structured for improved mechanical strength and improved current flow.
To increase the performance, electrodes can be made of lead alloys containing 1–2% impurities of selenium, antimony, calcium, tin, and carbon. However, current collectors can also be made of pure lead to slightly increase cell capacity during deep discharge. TPPL batteries use pure lead collectors. |
|
| Electrode surface area and thickness | Smaller electrode surface areas and thicker plates provide deeper discharge. They are used in «deep-cycle» lead-acid batteries.
Larger electrode surface area and thinner plates provide higher discharge current. They are found in starting, lighting, and ignition (SLI) batteries. TPPL batteries are a good example. |
|
Is there room for improvement in TPPL batteries?
There are serious fundamental restrictions on further lead-acid technology improvements, due to the chemical processes inside the battery.
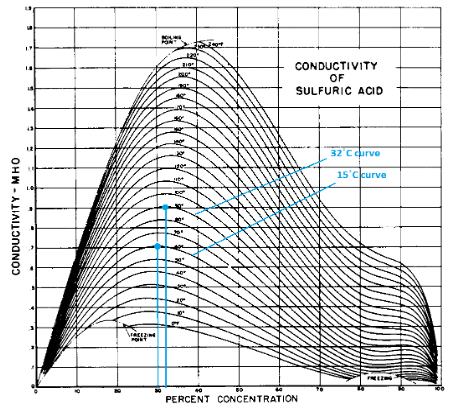
of conductivity vs. concentration (in %).
* The electrical conductivity of a sulfuric acid (H2SO4) solution is highest at a concentration of 30–33%, but it decreases sharply at temperatures above +32 °C and below +15 °C.
Concentrated sulfuric acid has a lower electrical conductivity than dilute sulfuric acid. This is due to the presence of fewer H+ and SO42- ions in concentrated form.
* The preferred depth of discharge (DOD) is only 50%
Figure 2. Constant temperature curves of conductivity vs. concentration (in %).When discharging a battery, the formation of PbSO4 sulfate on the electrodes is a natural process. As the depth of discharge (DOD) increases, sulfation increases as well. A DOD of 50% DOD is preferable, but 80% DOD is the maximum safe discharge. Increased sulfation is very harmful because instead of loose, fine sulfate crystals, a continuous dense layer of large crystals is formed on the electrode surface. With deep discharge, the volume of plates increases greatly, which may lead to deformation and destruction of the plates.
How does today’s TPPL technology compare to lithium-ion?
Being an improved version of the AGM, TPPL battery technology has several obvious improvements over a regular flooded lead-acid battery (FLA):
- TPPL plates are thinner, allowing for more plates and a larger reactive surface area, resulting in lower internal resistance and lower energy losses. This also enables quicker recharging and higher current delivery with lower voltage drop.
- There is no need to top-up the water daily.
- Gas emissions are lower.
- The cycle life is longer , especially when subjected to repeated micro cycles of discharging followed by partial opportunity charging.
Nevertheless, even with the most recent improvements, TPPL batteries remain inferior to LFP lithium batteries:
- Lag in energy densityCurrently, the lead plates account for an estimated 40–60% of the weight of an average lead-acid battery. A detailed comparison of AGM and LFP can be found in our article “Absorbed Glass Mat (AGM) vs Li-ion Battery”. This means TPPL batteries cannot compete with lithium for demanding applications, where more energy is needed to keep multi-shift operations running smoothly with one battery per truck.
- Lower performance at sub-zero temperaturesThe maximum conductivity of a TPPL battery at -6 °F is half the conductivity when the electrolyte temperature is 60 °F. Lithium batteries withstand harsher conditions and offer special insulation and inbuilt thermo-regulation, as in the OneCharge FROST package for extreme ambient temperatures.
Possible further TPPL technology developments
There are several ways in which the design of TPPL batteries may be improved to enhance performance:
- Thinner electrodes and increasing grid perforation (adding hole area to the total surface area).Changes to the electrode plate design and the use of powders increase the electrolyte-electrode contact surface, thereby increasing the electrical capacity of the battery. Plates are made by pasting lead dioxide (PbO2) on the positive electrode (cathode), and the lead (Pb) mass on the negative electrode (anode) while both electrodes have a perforated grid shape. The higher the degree of perforation, the more powder can be added.N.B. A very thin grid structure may be too fragile. Other metals in small quantities would need to be alloyed with lead for added strength and improved electrical properties.
- For collector grids, the use of lighter materials may significantly decrease plate weight and therefore increase specific energy (Wh/kg). Such materials must not react with sulfuric acid and be able to tightly bond to lead dioxide and lead coatings.Titanium is 2.5 times lighter than lead and could well be used for plate production, and there is some data available for titanium-based positive grids. According to The Elements: CRC Handbook of Chemistry and Physics (103rd Edition, 2022-2023), titanium’s specific gravity at 20 °C is 4.54 tons/m3, while the same parameter for lead is 11.35 tons/m3.
- Carbon as an additive for plate coatings may be used for both positive and negative electrodes to help prevent the formation of a passive lead sulfate (PbSO4) layer and increase the proportion of electrode mass involved in reactions.
The main constraint for all three opportunities is the cost of production. Adding complex and expensive components into the most inexpensive technology will make TPPL products unreasonably expensive; they will be competing at a similar price level with more efficient lithium and the new battery technologies, such as sodium-ion.
Conclusions
The next generation of TPPL batteries will directly compete with the newest lithium-ion and sodium-ion batteries, and the increasing complexity of the manufacturing process will inevitably increase the final price for end users. While TPPL technology faces an ever-increasing cost of manufacturing, with a lower starting point, lithium batteries are getting cheaper with increasing scale and competition.
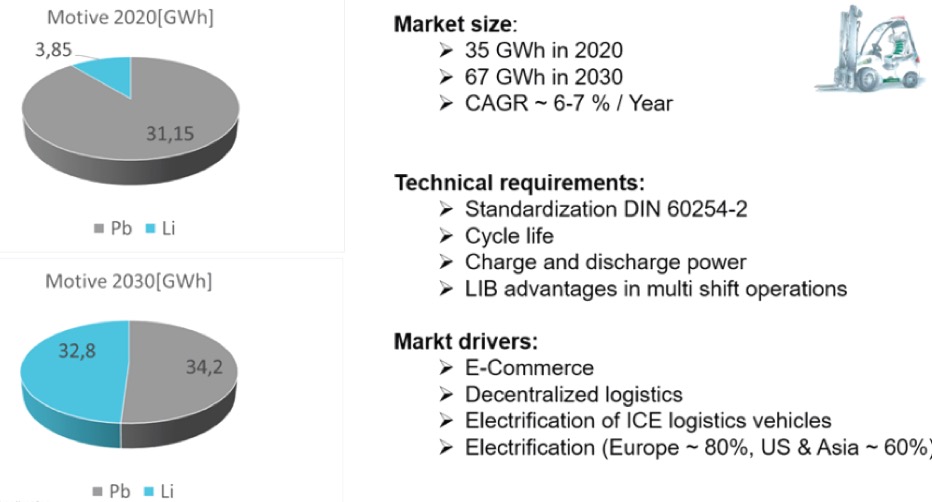
The present battery demand forecast in the material handling equipment (MHE) industry is impressive and is growing along with the logistics industry and an ever-increasing share of electric trucks (over 70% today).
Source: EUROBAT 2030 Battery Innovation Roadmap, version 2.0In June 2022, EUROBAT, a leading European association for advanced rechargeable batteries, published its 2030 Battery Innovation Roadmap, version 2.0. According to their projections, the demand for lithium-ion batteries will increase about 10-fold in this decade (from 3.85 GWh in 2020 to 32.8 GWh in 2030), and the market share of lead-acid will drop to about 50%, from today’s dominant position of around 90%.