 The diversity in forklift lithium battery cell design, chemistry composition, and form is what sets apart industrial lithium brands on the most basic level.
The diversity in forklift lithium battery cell design, chemistry composition, and form is what sets apart industrial lithium brands on the most basic level.
It is increasingly hard to choose the right forklift battery, given the variety of equipment types, makes, and models designed for specific applications, work environments, and operation paces.
To see through the sales pitch and make an informed decision, you need to understand the differences among cells. On the surface, all forklift batteries look the same. This article will help you to better understand what battery packs are made of.
So what’s inside the “black box”?
Types of Lithium Batteries: Lithium Cell Design
The most technologically advanced, reliable manufacturers of lithium cells ensure that their industrial batteries provide top performance over a long cycle life by maintaining high standards in the following three main areas of cell technology:
– electrolyte
– cathode and anode materials
– membrane technology.
Electrolyte in Lithium Cells
The electrolyte plays a key role in transporting the positive lithium ions between the cathode and anode. The most commonly used electrolyte consists of a lithium salt, such as LiPF6, organic solvents, and proprietary additives to improve stability and prevent dendrite formation and degradation of the solution.
These requirements are especially important in high-energy-density industrial battery applications:
– stability in high-current-discharge applications
– longer cycle life
– high conductivity
– high rate of discharge
– improved performance over a wide range of temperatures
– excellent thermal and hydrolytic stability
– excellent anti-overcharging stability
– high-purity LiPF6: >99.5%; free acid <50 ppm, water <20 ppm.
Cathode and Anode Materials of Lithium-Ion Cells
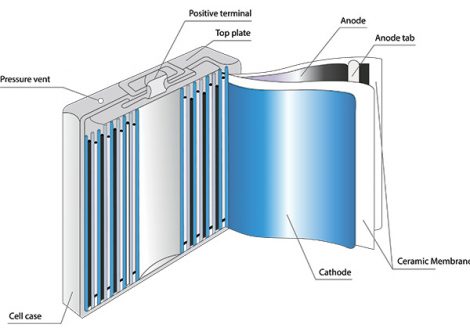 There are multiple cathode materials to choose from within the field of Li-ion technology. Originally, the primary active component of the cathode was lithium cobalt oxide. Today, cobalt is frequently substituted out with iron (LFP), nickel, manganese, and aluminum (NMC, NCA). Cathode materials require extremely high purity levels and must be almost entirely free of unwanted impurities, such as vanadium and sulfur.
There are multiple cathode materials to choose from within the field of Li-ion technology. Originally, the primary active component of the cathode was lithium cobalt oxide. Today, cobalt is frequently substituted out with iron (LFP), nickel, manganese, and aluminum (NMC, NCA). Cathode materials require extremely high purity levels and must be almost entirely free of unwanted impurities, such as vanadium and sulfur.
The most demanding battery applications require that the cells’ anode and cathode electrodes:
– provide high energy density
– ensure stability in high pulse discharge rate
– allow for fast charging speeds
– demonstrate resiliency against natural degradation.
Ceramic Membrane Used in Lithium-Ion Cells
An electrochemical cell consists of an anode and a cathode that are separated by an ion-permeable or ion-conductive membrane—the separator—as one of the main components.
State-of-the-art lithium cell technology uses a ceramic-coated separator, which improves cell performance at high temperatures and enhances battery safety.
Lithium cell membranes’ physical properties demonstrate:
– high thermal stability, porosity, and tortuosity of the pores
– effective ionic conductivity
– full compatibility with the combination of anode and cathode materials.
Read more about lithium cell design.
Types of Lithium Batteries: Lithium Cell Chemistry.
Lithium cells are named after the chemical composition of their cathode material.
The biggest impact on the specs of today’s commercially available batteries is made by the chemistry of their cathode materials. The best-known active component of the cathode is cobalt, widely used in batteries for electronics and EVs. Today, battery manufacturers using cobalt are facing serious supply-chain sustainability issues (like unethical mining practices, including the use of child labor). Cobalt is frequently replaced by iron (LFP), nickel, manganese, and aluminum.
Why LFP is the Best Choice for Material Handling Operations
Of all the various types of lithium-ion batteries, three cell chemistry types widely used in on- and off-highway electric vehicles: lithium iron phosphate, or lithium ferro phosphate (LFP); lithium nickel manganese cobalt oxide (NMC); and lithium nickel cobalt aluminum oxide (NCA).
A battery’s longevity, or its cycle life, depends on three main factors:
- chemical composition of cathode materials
- ambient temperature of operation
- depth of discharge.
All batteries degrade with usage, decreasing their Ah capacity with each charge/discharge cycle. In material handling, batteries usually become unusable when they drop below 80% of their nominal capacity.
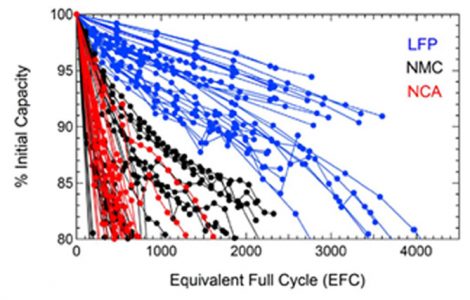
This graph shows the results of recent independent degradation tests of the aforementioned three types of cells with different chemistry, under
equal conditions of temperature and depth of discharge. LFP lithium batteries exhibit superior performance compared to NMC—they offer a longer lifespan and are generally less expensive. Lithium nickel cobalt aluminum oxide (NCA) batteries performed similarly to or worse than NMC.
The tests were performed at Sandia National Laboratories as “part of a broader effort to determine and characterize the safety and reliability of commercial Li-ion cells.”
Read more about the comparative characteristics of LFP, NMC, and NCA lithium cells.
Types of lithium batteries: lithium cell format
Main lithium battery pack components
Currently, there is no standardized design for a lithium-ion battery (LIB). The battery cell type is selected according to a user’s needs, which ultimately influences the design of the battery module. The present LIB market most often provides a 3-tier battery concept to customers: cell, module, and pack.
Cell shapes
Battery cells are designed in different form-factors and shapes: cylindrical, prismatic, and pouch cell. The inner structure and the electrode-separator compound differ in terms of the material dimensions and the manufacturing processes used.
 Prismatic lithium cell
Prismatic lithium cell Cylindrical lithium cell
Cylindrical lithium cell Pouch lithium cell
Pouch lithium cellCylindrical cells
Cylindrical cells consist of a sheet-like battery anode and cathode and separator that are sandwiched, rolled up, and packed into a cylinder-shaped can. This type of cell was one of the first mass-produced types and is still very popular. Cells feature multiple rows with arresters on opposite sides.
Pouch cells
Pouch cells do not have a rigid enclosure, using sealed, flexible foil as the cell container instead. The electrode and separator layers of a pouch cell are stacked. With pouch cells, the designer should allocate enough space for possible swelling of the cell. Battery modules with pouch cells feature single-row cells with the arresters positioned either on the same or the opposite side.
Prismatic cells
Prismatic cells consist of large sheets of anodes, cathodes, and separators sandwiched, rolled up, and pressed to fit into a metal or hard-plastic housing in cubic form. The electrodes can also be assembled by layer stacking. Lithium prismatic cells feature either single-row or two-row modules while the arresters are always on the same side, regardless of the row.[1]
Applications
Cylindrical cells are widely used in power tools, toys, lamps, automotive applications, e-bikes, and some portable mobile energy systems. Pouch cells are widespread in smartphones, drones, laptops, wearable devices, etc. Finally, prismatic cells are widely used in electric vehicles, including off-highway industrial trucks and different types of forklifts, communication-based stations, grid energy storage, medical applications, etc.
Prismatic cells are the best choice for forklift batteries
The differences in battery technology today allow customers to choose the best fit for their applications. Lithium prismatic cells are the preferred technology for material handling equipment (MHE) for the following reasons:
- Hundreds of Ah nominal capacity. The technology provides the best ratio of power and energy per volume unit. It’s especially important in high-capacity, high-current and relatively low voltage batteries used in MHE.
- Optimal use of the available pack space. There are no space cavities between the cells. It allows the capacity of a battery pack to be maximized, while still leaving enough space for required extra weight, sealing, heater, an internal charger, or other battery upgrades in the limited space of a battery compartment.
- Contacts are strong enough for reliable bus-bar connections. This is a crucial safety factor for high-vibration operations, especially those involving cushion-tire types of lift trucks.
- Flexible battery weight. LIB pack weight is not a limitation for most MHE in terms of its range per charge (unlike passenger EVs). Forklifts operate mostly near their charging stations and their batteries are often engineered as a counterweight.
Read more about the technical comparison of cylindrical, pouch and prismatic battery cells.
[1] Eduard Gerlitz, Marvin Greifenstein, Janna Hofmann, Jürgen Fleischer, Analysis of the Variety of Lithium-Ion Battery Modules and the Challenges for an Agile Automated Disassembly System, Procedia CIRP, Volume 96,
2021, Pages 175-180, ISSN 2212-8271, https://doi.org/10.1016/j.procir.2021.01.071. (https://www.sciencedirect.com/science/article/pii/S2212827121000974)
 by Vladimir Karimov | Dezembro 1, 2021
by Vladimir Karimov | Dezembro 1, 2021